西游记第1回:灵根育孕源流出,心性修持大道生
诗曰:
混沌未分天地乱,茫茫渺渺无人见。
自从盘古破鸿蒙,开辟从兹清浊辨。
覆载群生仰至仁,发明万物皆成善。
欲知造化会元功,须看西游释厄传。
盖闻天地之数,有十二万九千六百岁为一元。将一元分为十二会,乃子、丑、寅、卯、辰、巳、午、未、申、酉、戌、亥之十二支也。每会该一万八百岁。且就一 日而论:子时得阳气,而丑则鸡鸣;寅不通光,而卯则日出;辰时食后,而巳则挨排;日午天中,而未则西蹉;申时晡而日落酉;戌黄昏而人定亥。譬于大数,若到 戌会之终,则天地昏蒙而万物否矣。再去五千四百岁,交亥会之初,则当黑暗,而两间人物俱无矣,故曰混沌。又五千四百岁,亥会将终,贞下起元,近子之会,而 复逐渐开明。邵康节曰:"冬至子之半,天心无改移。一阳初动处,万物未生时。"到此,天始有根。再五千四百岁,正当子会,轻清上腾,有日,有月,有星,有 辰。日、月、星、辰,谓之四象。故曰,天开于子。又经五千四百岁,子会将终,近丑之会,而逐渐坚实。易曰:"大哉乾元!至哉坤元!万物资生,乃顺承天。" 至此,地始凝结。再五千四百岁,正当丑会,重浊下凝,有水,有火,有山,有石,有土。水、火、山、石、土谓之五形。故曰,地辟于丑。又经五千四百岁,丑会 终而寅会之初,发生万物。历曰:"天气下降,地气上升;天地交合,群物皆生。"至此,天清地爽,阴阳交合。再五千四百岁,正当寅会,生人,生兽,生禽,正 谓天地人,三才定位。故曰,人生于寅。
感盘古开辟,三皇治世,五帝定伦,世界之间,遂分为四大部洲:曰东胜神洲,曰西牛贺洲,曰南赡部 洲,曰北俱芦洲。这部书单表东胜神洲。海外有一国土,名曰傲来国。国近大海,海中有一座山,唤为花果山。此山乃十洲之祖脉,三岛之来龙,自开清浊而立,鸿 蒙判后而成。真个好山!有词赋为证。赋曰:
势镇汪洋,威宁瑶海。势镇汪洋,潮涌银山鱼入穴;威宁瑶海,波翻雪浪蜃离渊。木火方隅高积上,东 海之处耸崇巅。丹崖怪石,削壁奇峰。丹崖上,彩凤双鸣;削壁前,麒麟独卧。峰头时听锦鸡鸣,石窟每观龙出入。林中有寿鹿仙狐,树上有灵禽玄鹤。瑶草奇花不 谢,青松翠柏长春。仙桃常结果,修竹每留云。一条涧壑藤萝密,四面原堤草色新。正是百川会处擎天柱,万劫无移大地根。
那座山,正当顶上,有 一块仙石。其石有三丈六尺五寸高,有二丈四尺围圆。三丈六尺五寸高,按周天三百六十五度;二丈四尺围圆,按政历二十四气。上有九窍八孔,按九宫八卦。四面 更无树木遮阴,左右倒有芝兰相衬。盖自开辟以来,每受天真地秀,日精月华,感之既久,遂有灵通之意。内育仙胞,一日迸裂,产一石卵,似圆球样大。因见风, 化作一个石猴,五官俱备,四肢皆全。便就学爬学走,拜了四方。目运两道金光,射冲斗府。惊动高天上圣大慈仁者玉皇大天尊玄穹高上帝,驾座金阙云宫灵霄宝 店,聚集仙卿,见有金光焰焰,即命千里眼、顺风耳开南天门观看。二将果奉旨出门外,看的真,听的明。须臾回报道:"臣奉旨观听金光之处,乃东胜神洲海东傲 来小国之界,有一座花果山,山上有一仙石,石产一卵,见风化一石猴,在那里拜四方,眼运金光,射冲斗府。如今服饵水食,金光将潜息矣。"玉帝垂赐恩慈 曰:"下方之物,乃天地精华所生,不足为异。"
那猴在山中,却会行走跳跃,食草木,饮涧泉,采山花,觅树果;与狼虫为伴,虎豹为群,獐鹿为友,猕猿为亲;夜宿石崖之下,朝游峰洞之中。真是"山中无甲子,寒尽不知年。"一朝天气炎热,与群猴避暑,都在松阴之下顽耍。你看他一个个:
跳树攀枝,采花觅果;抛弹子,邷么儿;跑沙窝,砌宝塔;赶蜻蜓,扑八蜡;参老天,拜菩萨;扯葛藤,编草帓;捉虱子,咬又掐;理毛衣,剔指甲;挨的挨,擦的擦;推的推,压的压;扯的扯,拉的拉,青松林下任他顽,绿水涧边随洗濯。
一 群猴子耍了一会,却去那山涧中洗澡。见那股涧水奔流,真个似滚瓜涌溅。古云:"禽有禽言,兽有兽语。"众猴都道:"这股水不知是那里的水。我们今日赶闲无 事,顺涧边往上溜头寻看源流,耍子去耶!"喊一声,都拖男挈女,呼弟呼兄,一齐跑来,顺涧爬山,直至源流之处,乃是一股瀑布飞泉。但见那:
一派白虹起,千寻雪浪飞;
海风吹不断,江月照还依。
冷气分青嶂,馀流润翠微;
潺湲名瀑布,真似挂帘帷。
众猴拍手称扬道:"好水!好水!原来此处远通山脚之下,直接大海之波。"又道:"那一个有本事的,钻进去寻个源头出来,不伤身体者,我等即拜他为王。"连呼了三声,忽见丛杂中跳出一名石猴,应声高叫道:"我进去!我进去!"好猴!也是他:
今日芳名显,时来大运通;
有缘居此地,王遣入仙宫。
你看他瞑目蹲身,将身一纵,径跳入瀑布泉中,忽睁睛抬头观看,那里边却无水无波,明明朗朗的一架桥梁。他住了身,定了神,仔细再看,原来是座铁板桥。桥 下之水,冲贯于石窍之间,倒挂流出去,遮闭了桥门。却又欠身上桥头,再走再看,却似有人家住处一般,真个好所在。但见那:
翠藓堆蓝,白云浮玉,光摇片片烟霞。虚窗静室,滑凳板生花。乳窟龙珠倚挂,萦回满地奇葩。锅灶傍崖存火迹,樽罍靠案见肴渣。石座石床真可爱,石盆石碗更堪夸。又见那一竿两竿修竹,三点五点梅花。几树青松常带雨,浑然相个人家。
看罢多时,跳过桥中间,左右观看,只见正当中有一石碣。碣上有一行楷书大字,镌着"花果山福地,水帘洞洞天。"石猴喜不自胜,急抽身往外便走,复瞑目蹲 身,跳出水外,打了两个呵呵道:"大造化!大造化!"众猴把他围住,问道:"里面怎么样?水有多深?"石猴道:"没水!没水!原来是一座铁板桥。桥那边是 一座天造地设的家当。"众猴道:"怎见得是个家当?"石猴笑道:"这股水乃是桥下冲贯石桥,倒挂下来遮闭门户的。桥边有花有树,乃是一座石房。房内有石 窝、石灶、石碗、石盆、石床、石凳。中间一块石碣上,镌着'花果山福地,水帘洞洞天。'真个是我们安身之处。里面且是宽阔,容得千百口老小。我们都进去住 也,省得受老天之气。这里边:
刮风有处躲,下雨好存身。
霜雪全无惧,雷声永不闻。
烟霞常照耀,祥瑞每蒸熏。
松竹年年秀,奇花日日新。"
众猴听得,个个欢喜,都道:"你还先走,带我们进去,进去!"石猴却又瞑目蹲身,往里一跳,叫道:"都随我进来!进来!"那些猴有胆大的,都跳进去了; 胆小的,一个个伸头缩颈,抓耳挠腮,大声叫喊,缠一会,也都进去了。跳过桥头,一个个抢盆夺碗,占灶争床,搬过来,移过去,正是猴性顽劣,再无一个宁时, 只搬得力倦神疲方止。石猿端坐上面道:"列位呵,'人而无信,不知其可。'你们才说有本事进得来,出得去,不伤身体者,就拜他为王。我如今进来又出去,出 去又进来,寻了这一个洞天与列位安眠稳睡,各享成家之福,何不拜我为王?"众猴听说,即拱伏无违。一个个序齿排班,朝上礼拜,都称"千岁大王"。自此,石 猴高登王位,将"石"字儿隐了,遂称美猴王。有诗为证。诗曰:
三阳交泰产群生,仙石胞含日月精。
借卵化猴完大道,假他名姓配丹成。
内观不识因无相,外合明知作有形。
历代人人皆属此,称王称圣任纵横。
美猴王领一群猿猴、猕猴、马猴等,分派了君臣佐使,朝游花果山,暮宿水帘洞,合契同情,不入飞鸟之丛,不从走兽之类,独自为王,不胜欢乐。是以:
春采百花为饮食,夏寻诸果作生涯。
秋收芋栗延时节,冬觅黄精度岁华。
美猴王享乐天真,何期有三五百载。一日,与群猴喜宴之间,忽然忧恼,堕下泪来。众猴慌忙罗拜道:"大王何为烦恼?"猴王道:"我虽在欢喜之时,却有一点 儿远虑,故此烦恼。"众猴又笑道:"大王好不知足!我等日日欢会,在仙山福地,古洞神州,不伏麒麟辖,不伏凤凰管,又不伏人间王位所拘束,自由自在,乃无 量之福,为何远虑而忧也?"猴王道:"今日虽不归人王法律,不惧禽兽威服,将来年老血衰,暗中有阎王老子管着,一旦身亡,可不枉生世界之中,不得久住天人 之内?"众猴闻此言,一个个掩面悲啼,俱以无常为虑。
只见那班部中,忽跳出一个通背猿猴,厉声高叫道:"大王若是这般远虑,真所谓道心 开发也!如今五虫之内,惟有三等名色,不伏阎王老子所管。"猴王道:"你知那三等人?"猿猴道:"乃是佛与仙与神圣三者,躲过轮回,不生不灭,与天地山川 齐寿。"猴王道:"此三者居于何所?"猿猴道:"他只在阎浮世界之中,古洞仙山之内。"猴王闻之,满心欢喜,道:"我明日就辞汝等下山,云游海角,远涉天 涯,务必访此三者,学一个不老长生,常躲过阎君之难。"噫!这句话:
顿教跳出轮回网,致使齐天大圣成。
众猴鼓掌称扬,都道:"善哉!善哉!我等明日越岭登山,广寻些果品,大设筵宴送大王也。"
次日,众猴果去采仙桃,摘异果,刨山药,劚黄精,芝兰香蕙,瑶草奇花,般般件件,整整齐齐,摆开石凳石桌,排列仙酒仙肴。但见那:
金 丸珠弹,红绽黄肥。金丸珠弹腊樱桃,色真甘美;红绽黄肥熟梅子,味果香酸。鲜龙眼,肉甜皮薄;火荔枝,核小囊红。林檎碧实连枝献,枇杷缃苞带叶擎。兔头梨 子鸡心枣,消渴除烦更解酲。香桃烂杏,美甘甘似玉液琼浆;脆李杨梅,酸荫荫如脂酸膏酪。红囊黑子熟西瓜,四瓣黄皮大柿子。石榴裂破,丹砂粒现火晶珠;芋栗 剖开,坚硬肉团金玛瑙。胡桃银杏可传茶,椰子葡萄能做酒。榛松榧柰满盘盛,橘蔗柑橙盈案摆。熟煨山药,烂煮黄精,捣碎茯苓并薏苡,石锅微火漫炊羹。人间纵 有珍馐味,怎比山猴乐更宁?
群猴尊美猴王上坐,各依齿肩排于下边,一个个轮流上前,奉酒,奉花,奉果,痛饮了一日。次日,美猴王早起, 教:"小的们,替我折些枯松,编作筏子,取个竹竿作篙,收拾些果品之类,我将去也。"果独自登筏,尽力撑开,飘飘荡荡,径向大海波中,趁天风,来渡南赡部 洲地界。这一去,正是那:
天产仙猴道行隆,离山驾筏趁天风。
飘洋过海寻仙道,立志潜心建大功。
有分有缘休俗愿,无忧无虑会元龙。
料应必遇知音者,说破源流万法通。
也 是他运至时来,自登木筏之后,连日东南风紧,将他送到西北岸前,乃是南赡部洲地界。持篙试水,偶得浅水,弃了筏子,跳上岸来,只见海边有人捕鱼、打雁、挖 蛤、淘盐。他走近前,弄个把戏,妆个□【上左"齿"右"可",下"女"】虎,吓得那些人丢筐弃网,四散奔跑。将那跑不动的拿住一个,剥了他衣裳,也学人穿 在身上,摇摇摆摆,穿州过府,在市尘中,学人礼,学人话。朝餐夜宿,一心里访问佛仙神圣之道,觅个长生不老之方。见世人都是为名为利之徒,更无一个为身命 者。正是那:
争名夺利几时休?早起迟眠不自由!
骑着驴骡思骏马,官居宰相望王侯。
只愁衣食耽劳碌,何怕阎君就取勾?
继子荫孙图富贵,更无一个肯回头!
猴王参访仙道,无缘得遇。在于南赡部洲,串长城,游小县,不觉八九年馀。忽行至西洋大海,他想着海外必有神仙。独自个依前作筏,又飘过西海,直至西牛贺洲地界。登岸偏访多时,忽见一座高山秀丽,林麓幽深。他也不怕狼虫,不惧虎豹,登山顶上观看。果是好山:
千峰开戟,万仞开屏。日映岚光轻锁翠,雨收黛色冷含青。枯藤缠老树,古渡界幽程。奇花瑞草,修竹乔松。修竹乔松,万载常青欺福地;奇花瑞草,四时不谢赛蓬瀛。幽鸟啼声近,源泉响溜清。重重谷壑芝兰绕,处处巉崖苔藓生。起伏峦头龙脉好,必有高人隐姓名。
正观看间,忽闻得林深之处,有人言语,急忙趋步,穿入林中,侧耳而听,原来是歌唱之声。歌曰:
"观棋柯烂,伐木丁丁,云边谷口徐行,卖薪沽酒,狂笑自陶情。苍迳秋高,对月枕松根,一觉天明。认旧林,登崖过岭,持斧断枯藤。收来成一担,行歌市上,易米三升。更无些子争竞,时价平平,不会机谋巧算,没荣辱,恬淡延生。相逢处,非仙即道,静坐讲黄庭。"
美猴王听得此言,满心欢喜道:"神仙原来藏在这里!"急忙跳入里面,仔细再看,乃是一个樵子,在那里举斧砍柴。但看他打扮非常:
头上戴箬笠,乃是新笋初脱之箨。身上穿布衣,乃是木绵捻就之纱。腰间系环绦,乃是老蚕口吐之丝。足下踏草履,乃是枯莎搓就之爽。手执衠钢斧,担挽火麻绳。扳松劈枯树,争似此樵能!
猴王近前叫道:"老神仙!弟子起手。"那樵汉慌忙丢了斧,转身答礼道:"不当人!不当人!我拙汉衣食不全,怎敢当'神仙'二字?"猴王道:"你不是神 仙,如何说出神仙的话来?"樵夫道:"我说甚么神仙话?"猴王道:"我才来至林边,只听的你说:'相逢处非仙即道,静坐讲黄庭。'黄庭乃道德真言,非神仙 而何?"樵夫笑道:"实不瞒你说,这个词名做满庭芳,乃一神仙教我的。那神仙与我舍下相邻。他见我家事劳苦,日常烦恼,教我遇烦恼时,即把这词儿念念。一 则散心,二则解困。我才有些不足处思虑,故此念念。不期被你听了。"猴王道:"你家既与神仙相邻,何不从他修行?学得个不老之方?却不是好?"樵夫道:" 我一生命苦,自幼蒙父母养育至八九岁,才知人事,不幸父丧,母亲居孀。再无兄弟姊妹,只我一人,没奈何,早晚侍奉。如今母老,一发不敢抛离。却又田园荒 芜,衣食不足,只得斫两束柴薪,挑向市尘之间,货几文钱,籴几升米,自炊自造,安排些茶饭,供养老母,所以不能修行。"
猴王道:"据你 说起来,乃是一个行孝的君子,向后必有好处。但望你指与我那神仙住处,却好拜访去也。"樵夫道:"不远,不远。此山叫做灵台方寸山。山中有座斜月三星洞。 那洞中有一个神仙,称名须菩提祖师。那祖师出去的徒弟,也不计其数,见今还有三四十人从他修行。你顺那条小路儿,向南行七八里远近,即是他家了。"猴王用 手扯住樵夫道:"老兄,你便同我去去。若还得了好处,决不忘你指引之恩。"樵夫道:"你这汉子,甚不通变。我方才这般与你说了,你还不省?假若我与你去 了,却不误了我的生意?老母何人奉养?我要斫柴,你自去,自去。"
猴王听说,只得相辞。出深林,找上路径,过一山坡,约有七八里远,果然望见一座洞府。挺身观看,真好去处!但见:
烟 霞散彩,日月摇光。千株老柏,万节修篁。千株老柏,带雨半空青冉冉;万节修篁,含烟一壑色苍苍。门外奇花布锦,桥边瑶草喷香。石崖突兀青苔润,悬壁高张翠 藓长。时闻仙鹤唳,每见凤凰翔。仙鹤唳时,声振九皋霄汉远;凤凰翔起,翎毛五色彩云光。玄猿白鹿随隐见,金狮玉象任行藏。细观灵福地,真个赛天堂!
又见那洞门紧闭,静悄悄杳无人迹。忽回头,见崖头立一石牌,约有三丈馀高、八尺馀阔,上有一行十个大字,乃是"灵台方寸山,斜月三星洞"。美猴王十分欢喜道:"此间人果是朴实。果有此山此洞。"看勾多时,不敢敲门。且去跳上松枝梢头,摘松子吃了顽耍。
少顷间,只听得呀的一声,洞门开处,里面走出一个仙童,真个丰姿英伟,像貌清奇,比寻常俗子不同。但见他:
髽髻双丝绾,宽袍两袖风。
貌和身自别,心与相俱空。
物外长年客,山中永寿童。
一尘全不染,甲子任翻腾。
那童子出得门来,高叫道:"甚么人在此搔扰?"猴王扑的跳下树来,上前躬身道:"仙童,我是个访道学仙之弟子,更不敢在此搔扰。"仙童笑道:"你是个访 道的么?"猴王道:"是。"童子道:"我家师父,正才下榻,登坛讲道。还未说出原由,就教我出来开门。说:'外面有个修行的来了,可去接待接待。'想必就 是你了?"猴王笑道:"是我,是我。"童子道:"你跟我进来。"
这猴王整衣端肃,随童子径入洞天深处观看:一层层深阁琼楼,一进进珠宫贝阙,说不尽那静室幽居,直至瑶台之下。见那菩提祖师端坐在台上,两边有三十个小仙侍立台下。果然是:
大觉金仙没垢姿,西方妙相祖菩提;
不生不灭三三行,全气全神万万慈。
空寂自然随变化,真如本性任为之;
与天同寿庄严体,历劫明心大法师。
美猴王一见,倒身下拜,磕头不计其数,口中只道:"师父!师父!我弟子志心朝礼!志心朝礼!"祖师道:"你是那方人氏?且说个乡贯姓名明白,再拜。"猴 王道:"弟子东胜神洲傲来国花果山水帘洞人氏。"祖师喝令:"赶出去!他本是个撒诈捣虚之徒,那里修甚么道果!"猴王慌忙磕头不住道:"弟子是老实之言, 决无虚诈。"祖师道:"你既老实,怎么说东胜神洲?那去处到我这里,隔两重大海,一座南赡部洲,如何就得到此?"猴王叩头道:"弟子飘洋过海,登界游方, 有十数个年头,方才访到此处。"
祖师道:"既是逐渐行来的也罢。你姓甚么?"猴王又道:"我无性。人若骂我,我也不恼;若打我,我也不 嗔,只是陪个礼儿就罢了。一生无性。"祖师道:"不是这个性。你父母原来姓甚么?"猴王道:"我也无父母。"祖师道:"既无父母,想是树上生的?"猴王 道:"我虽不是树生,却是石里长的。我只记得花果山上有一块仙石,其年石破,我便生也。"祖师闻言,暗喜道:"这等说,却是天地生成的。你起来走走我 看。"猴王纵身跳起,拐呀拐的走了两遍。祖师笑道:"你身躯虽是鄙陋,却像个食松果的猢狲。我与你就身上取个姓氏,意思教你姓'猢'。猢字去了个兽傍,乃 是古月。古者,老也;月者,阴也。老阴不能化育,教你姓'狲'倒好。狲字去了兽傍,乃是个子系。子者,儿男也;系者,婴细也。正合婴儿之本论。教你姓'孙 '罢。"猴王听说,满心欢喜,朝上叩头道:"好!好!好!今日方知姓也。万望师父慈悲!既然有姓,再乞赐个名字,却好呼唤。"祖师道:"我门中有十二个 字,分派起名到你乃第十辈之小徒矣。"猴王道:"那十二个字?"祖师道:"乃广、大、智、慧、真、如、性、海、颖、悟、圆、觉十二字。排到你,正当'悟' 字。与你起个法名叫做'孙悟空'好么?"猴王笑道:"好!好!好!自今就叫做孙悟空也!"正是:
鸿蒙初辟原无姓,打破顽空须悟空。
毕竟不知向后修些甚么道果,且听下回分解。
Pang Zi Jing
Monday, October 25, 2010
Sunday, October 24, 2010
Genting Highland

Genting Highlands
From Wikipedia, the free encyclopedia
Jump to: navigation, search
| Resorts World Genting | |
 | |
 Resorts World Genting | |
| Facts and statistics | |
|---|---|
| Opening date | 1965 |
| Total gaming space | Over 200,000 sq ft (19,000 m2) |
| Casino type | Land-Based |
| Owner | Genting Group |
| Website | http://www.rwgenting.com |
Contents[hide] |
[edit] History
In 2006, the resort had 18.4 million visitors.[1] Resorts World Genting was founded by the late Lim Goh Tong (Tan Sri) in the late 1960s. Currently, this resort is being lead by Lim Goh Tong's son, Lim Kok Thay (Tan Sri) who is also the current president and CEO of Star Cruises. Resorts World Genting is the sister resort to the Resorts World Sentosa and Resorts World Manila.[edit] Attractions
It is sometimes informally known as the Las Vegas of Malaysia, dubbed the "City of Entertainment" as it has the only legal land-based casino, Casino de Genting in the country and is run by Genting Malaysia Bhd, a subsidiary of Genting Group.[edit] Hotels
Resorts World Genting has six hotels. In 2006 Guinness World Records listed the First World Hotel as the world's largest hotel with a total of 6,118 rooms.[2][edit] Dining
- Bakery - Pastries, sandwiches & desserts
- Coffee Terrace - Asian and Western buffet
- Genting Palace Restaurant - Cantonese cuisine
- Hainan Kitchen - Hainanese cuisine
- Imperial Rama - Fine Dining Thai-Chinese cuisine
- Ming Ren Restaurant - Xinjiang cuisine
- The Olive - Fine Dining Western cuisine
- VIP Restaurant - Thai-Chinese cuisine
[edit] Nightlife
- Safari
- Cloud 9
- All Sports Bar
- Patio Bar & Lounge
[edit] Theme Parks
The resort has three theme parks which are Genting Outdoor Theme Park, First World Indoor Theme Park and Water Park. There are over 20 signature attractions which include:- Flying Coaster
- Genting Sky Venture
- Haunted House
- Ripley's Believe It or Not! Museum
- SnowWorld
- Space Shot
[edit] Genting Skyway
Genting Skyway, located at the Kuala Kubu Bharu, Selangor side of Genting, Malaysia, is a monocable gondola lift serving the Genting Highlands Resort. Its lower station is located near Gohtong Jaya, and its upper station is located at Highlands Hotel of Genting Highlands Resort.It was officially opened in 1997 by the former prime minister of Malaysia, Mahathir bin Mohammad.
[edit] Amenities
The resort has five performance venues.- Arena of Stars
- Genting International Showroom
- Genting International Convention Centre
- Pavilion Hall
- First World Plaza
[edit] Genting International Convention Centre
GICC consists of a Grand Ballroom, 6 meeting rooms at first tower block & 12 meeting rooms at second tower block, supported by an aesthetically designed and spacious pre-function foyer, a well-equipped business centre and VIP / Media reception rooms.The Grand Ballroom is the largest column-free hall in the country and can accommodate up to 2,000 banquet-style and up to 4,200 in theatre-style seating, and the entire area can be split up conveniently into two to four sections according to clients' needs.
[edit] Events
[edit] Awards ceremonies
The resort hosts several award ceremonies at the Arena of Stars, with capacity of 6,000 people.- IIFA Awards 2002 - hosted by Amitabh Bachchan
- Zee Cine Awards 2007
- Lux Style Awards 2007
- MTV Asia Awards 2008 - hosted by Jared Leto
[edit] Concerts
- X-Pax 'XLive Festival' 2008 - music festival headlined by Missy Elliott
[edit] Asian Artists | [edit] Western Artists Boyz II Men at Arena of Stars |
[edit] Television Shows
Genting also plays host to a range of popular TV shows being filmed on site including:[edit] Sports
Genting TrailblazerAn annual 14 km adventure racing and trail running marathon through the rainforest.
Tour de Langkawi
Genting Highlands serve as the finish point of a stage in the Tour de Langkawi cycling race. It is one of the longest (30 km) climbs featured in a cycling event.
[edit] Awards
Over the years, Genting Highlands have received numerous awards for their excellence including:- World's Leading Casino Resort, World Travel Awards 2007/2008/2009
- Best Resort, TTG Travel Awards 2007/2008/2009
- The BrandLaureate Awards, The Best Brands In Leisure And Hospitality 2006 - 2009
- Top 1 Most Valuable Brands, Asia's Most Valuable Brands 2007/2008/2009
- The World's Largest Hotel, Guinness World Records 2006/2007/2008/2009
- Malaysia Spa & Wellness Awards 2007/2008/2009 - Best Resort Spa
Electron Transport Chain
Electron transport chain
From Wikipedia, the free encyclopedia
Jump to: navigation, search
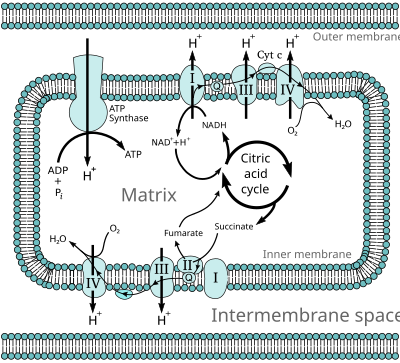
The electron transport chain in the mitochondrion is the site of oxidative phosphorylation in eukaryotes. The NADH and succinate generated in the citric acid cycle are oxidized, providing energy to power ATP synthase.
In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH and a transfer of H+ ions. NADPH is used as an electron donor for carbon fixation. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that drives the transfer of H+ ions. While some bacteria have electron transport chains similar to those in chloroplasts or mitochondria, other bacteria use different electron donors and acceptors. Both the respiratory and photosynthetic electron transport chains are major sites of premature electron leakage to oxygen, thus being major sites of superoxide production and drivers of oxidative stress.
Contents[hide] |
[edit] Background
The electron transport chain consists of a spatially separated series of redox reactions in which electrons are transferred from a donor molecule to an acceptor molecule. The underlying force driving these reactions is the Gibbs free energy of the reactants and products. The Gibbs free energy is the energy available ("free") to do work. Any reaction that decreases the overall Gibbs free energy of a system will proceed spontaneously. The transfer of electrons proceeds from an electron donor to an acceptor.ATP synthase, an enzyme highly conserved among all domains of life, is powered by a transmembrane proton electrochemical gradient, which is the result of a series of redox reactions.[1][nb 1] The function of the electron transport chain is to produce this gradient.[2][nb 2]
The transmembrane electrochemical potential gradient may enable transport of molecules across membranes. It may also enable mechanical work, such as rotating bacterial flagella, or to produce ATP, which provides energy to power other cellular reactions. A small amount of ATP is available from substrate-level phosphorylation, for example, in glycolysis. In most organisms the majority of ATP is generated in electron transport chains, while only some obtain ATP by fermentation.[citation needed]
[edit] Electron transport chains in mitochondria
Most eukaryotic cells contain mitochondria, which produce ATP from products of the Krebs cycle, fatty acid oxidation, and amino acid oxidation. At the mitochondrial inner membrane, electrons from NADH and succinate pass through the electron transport chain to oxygen, which is reduced to water. This enzymatic series produces a proton gradient across the mitochondrial membrane, producing a thermodynamic state that has the potential to do work. A small percentage of electrons prematurely leak to oxygen, resulting in the formation of the toxic free-radical superoxide, a molecule thought to contribute to a number of diseases and aging.[edit] Mitochondrial redox carriers
Stylized representation of the ETC. Energy obtained through the transfer of electrons (black arrows) down the ETC is used to pump protons (red arrows) from the mitochondrial matrix into the intermembrane space, creating an electrochemical proton gradient across the mitochondrial inner membrane (IMM) called ΔΨ. This electrochemical proton gradient allows ATP synthase (ATP-ase) to use the flow of H+ through the enzyme back into the matrix to generate ATP from adenosine diphosphate (ADP) and inorganic phosphate. Complex I (NADH coenzyme Q reductase; labeled I) accepts electrons from the Krebs cycle electron carrier nicotinamide adenine dinucleotide (NADH), and passes them to coenzyme Q (ubiquinone; labeled UQ), which also receives electrons from complex II (succinate dehydrogenase; labeled II). UQ passes electrons to complex III (cytochrome bc1 complex; labeled III), which passes them to cytochrome c (cyt c). Cyt c passes electrons to Complex IV (cytochrome c oxidase; labeled IV), which uses the electrons and hydrogen ions to reduce molecular oxygen to water.
NADH → Complex I → Q → Complex III → cytochrome c → Complex IV → O2
↑
Complex II [edit] Complex I
In Complex I (NADH dehydrogenase, also called NADH:ubiquinone oxidoreductase; EC 1.6.5.3) two electrons are removed from NADH and transferred to a lipid-soluble carrier, ubiquinone (Q). The reduced product, ubiquinol (QH2) freely diffuses within the membrane, and Complex I translocates four protons (H+) across the membrane, thus producing a proton gradient. Complex I is one of the main sites at which premature electron leakage to oxygen occurs, thus being one of main sites of production of harmful superoxide.[citation needed]The pathway of electrons occurs as follows:
NADH is oxidized to NAD+, by reducing Flavin mononucleotide to FMNH2 in one two-electron step. FMNH2 is then oxidized in two one-electron steps, through a semiquinone intermediate. Each electron thus transfers from the FMNH2 to an Fe-S cluster, from the Fe-S cluster to ubiquinone (Q). Transfer of the first electron results in the free-radical (semiquinone) form of Q, and transfer of the second electron reduces the semiquinone form to the ubiquinol form, QH2. During this process, four protons are translocated from the mitochondrial matrix to the intermembrane space, creating a proton gradient that generates ATP through oxidative phosphorylation.
[edit] Complex II
In Complex II (succinate dehydrogenase; EC 1.3.5.1) additional electrons are delivered into the quinone pool (Q) originating from succinate and transferred (via FAD) to Q. Complex II consists of four protein subunits: SDHA, SDHB, SDHC, and SDHD. Other electron donors (e.g., fatty acids and glycerol 3-phosphate) also direct electrons into Q (via FAD).[edit] Complex III
In Complex III (cytochrome bc1 complex; EC 1.10.2.2) two electrons are removed from QH2 at the QO site and sequentially transferred to two molecules of cytochrome c, a water-soluble electron carrier located within the intermembrane space. The two other electrons sequentially pass across the protein to the Qi site where the quinone part of ubiquinone is reduced to quinol. A proton gradient is formed by two quinol (4H+4e-) oxidations at the Qo site to form one quinol (2H+2e-) at the Qi site. (in total six protons are translocated: two protons reduce quinone to quinol and four protons are released from two ubiquinol molecules). The bc1 complex does not 'pump' protons, but helps build the proton gradient by an asymmetric absorption/release of protons.[citation needed]When electron transfer is reduced (by a high membrane potential or respiratory inhibitors such as antimycin A), Complex III may leak electrons to molecular oxygen, resulting in superoxide formation.
[edit] Complex IV
In Complex IV (cytochrome c oxidase; EC 1.9.3.1) four electrons are removed from four molecules of cytochrome c and transferred to molecular oxygen (O2), producing two molecules of water. At the same time, four protons are translocated across the membrane, contributing to the proton gradient. The activity of cytochrome c is inhibited by cyanide.[edit] Coupling with oxidative phosphorylation
According to the chemiosmotic coupling hypothesis, proposed by Nobel Prize in Chemistry winner Peter D. Mitchell, the electron transport chain and oxidative phosphorylation are coupled by a proton gradient across the inner mitochondrial membrane. The efflux of protons from the mitochondrial matrix creates a electrochemical gradient (proton gradient). This gradient is used by the FOF1 ATP synthase complex to make ATP via oxidative phosphorylation. ATP synthase is sometimes described as Complex V of the electron transport chain.[citation needed] The FO component of ATP synthase acts as an ion channel that provides for a proton flux back into the mitochondrial matrix. This reflux releases free energy produced during the generation of the oxidized forms of the electron carriers (NAD+ and Q). The free energy is used to drive ATP synthesis, catalyzed by the F1 component of the complex.[citation needed]Coupling with oxidative phosphorylation is a key step for ATP production. However, in certain cases, uncoupling the two processes may be biologically useful. The uncoupling protein, thermogenin—present in the inner mitochondrial membrane of brown adipose tissue—provides for an alternative flow of protons back to the inner mitochondrial matrix. This alternative flow results in thermogenesis rather than ATP production and generates heat.[citation needed] Synthetic uncouplers (e.g., 2,4-dinitrophenol) also exist, and, at high doses, are lethal.[citation needed]
[edit] Summary
In the mitochondrial electron transport chain electrons move from an electron donor (NADH or QH2) to a terminal electron acceptor (O2) via a series of redox reactions. These reactions are coupled to the creation of a proton gradient across the mitochondrial inner membrane. There are three proton pumps: I, III, and IV. The resulting transmembrane proton gradient is used to make ATP via ATP synthase.The reactions catalyzed by Complex I and Complex III work roughly at equilibrium. This means that these reactions are readily reversible, by increasing the concentration of the products relative to the concentration of the reactants (for example, by increasing the proton gradient). ATP synthase is also readily reversible. Thus ATP can be used to build a proton gradient, which in turn can be used to make NADH. This process of reverse electron transport is important in many prokaryotic electron transport chains.[citation needed]
[edit] Electron transport chains in bacteria
In eukaryotes, NADH is the most important electron donor. The associated electron transport chain isNADH → Complex I → Q → Complex III → cytochrome c → Complex IV → O2 where Complexes I, III and IV are proton pumps, while Q and cytochrome c are mobile electron carriers. The electron acceptor is molecular oxygen.
In prokaryotes (bacteria and archaea) the situation is more complicated, because there are several different electron donors and several different electron acceptors. The generalized electron transport chain in bacteria is:
Donor Donor Donor
↓ ↓ ↓
dehydrogenase → quinone → bc1 → cytochrome
↓ ↓
oxidase(reductase) oxidase(reductase)
↓ ↓
Acceptor AcceptorNote that electrons can enter the chain at three levels: at the level of a dehydrogenase, at the level of the quinone pool, or at the level of a mobile cytochrome electron carrier. These levels correspond to successively more positive redox potentials, or to successively decreased potential differences relative to the terminal electron acceptor. In other words, they correspond to successively smaller Gibbs free energy changes for the overall redox reaction Donor → Acceptor.Individual bacteria use multiple electron transport chains, often simultaneously. Bacteria can use a number of different electron donors, a number of different dehydrogenases, a number of different oxidases and reductases, and a number of different electron acceptors. For example, E. coli (when growing aerobically using glucose as an energy source) uses two different NADH dehydrogenases and two different quinol oxidases, for a total of four different electron transport chains operating simultaneously.
A common feature of all electron transport chains is the presence of a proton pump to create a transmembrane proton gradient. Bacterial electron transport chains may contain as many as three proton pumps, like mitochondria, or they may contain only one or two. They always contain at least one proton pump.
[edit] Electron donors
In the present day biosphere, the most common electron donors are organic molecules. Organisms that use organic molecules as an energy source are called organotrophs. Organotrophs (animals, fungi, protists) and phototrophs (plants and algae) constitute the vast majority of all familiar life forms.Some prokaryotes can use inorganic matter as an energy source. Such organisms are called lithotrophs ("rock-eaters"). Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Lithotrophs have been found growing in rock formations thousands of meters below the surface of Earth. Because of their volume of distribution, lithotrophs may actually outnumber organotrophs and phototrophs in our biosphere.
The use of inorganic electron donors as an energy source is of particular interest in the study of evolution. This type of metabolism must logically have preceded the use of organic molecules as an energy source.
[edit] Dehydrogenases
Bacteria can use a number of different electron donors. When organic matter is the energy source, the donor may be NADH or succinate, in which case electrons enter the electron transport chain via NADH dehydrogenase (similar to Complex I in mitochondria) or succinate dehydrogenase (similar to Complex II). Other dehydrogenases may be used to process different energy sources: formate dehydrogenase, lactate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase, H2 dehydrogenase (hydrogenase), etc. Some dehydrogenases are also proton pumps; others simply funnel electrons into the quinone pool.Most of dehydrogenases are synthesized only when needed. Depending on the environment in which they find themselves, bacteria select different enzymes from their DNA library and synthesize only those that are needed for growth.Enzymes that are synthesized only when needed are said to be 'inducible'.
[edit] Quinone carriers
Quinones are mobile, lipid-soluble carriers that shuttle electrons (and protons) between large, relatively immobile macromolecular complexes embedded in the membrane. Bacteria use ubiquinone (the same quinone that mitochondria use) and related quinones such as menaquinone.[edit] Proton pumps
A proton pump is any process that creates a proton gradient across a membrane. Protons can be physically moved across a membrane; this is seen in mitochondrial Complexes I and IV. The same effect can be produced by moving electrons in the opposite direction. The result is the disappearance of a proton from the cytoplasm and the appearance of a proton in the periplasm. Mitochondrial Complex III uses this second type of proton pump, which is mediated by a quinone (the Q cycle).Some dehydrogenases are proton pumps; others are not. Most oxidases and reductases are proton pumps, but some are not. Cytochrome bc1 is a proton pump found in many, but not all, bacteria (it is not found in E. coli). As the name implies, bacterial bc1 is similar to mitochondrial bc1 (Complex III).
Proton pumps are the heart of the electron transport process. They produce the transmembrane electrochemical gradient that supplies energy to the cell.
[edit] Cytochrome electron carriers
Cytochromes are pigments that contain iron. They are found in two very different environments.Some cytochromes are water-soluble carriers that shuttle electrons to and from large, immobile macromolecular structures imbedded in the membrane. The mobile cytochrome electron carrier in mitochondria is cytochrome c. Bacteria use a number of different mobile cytochrome electron carriers.
Other cytochromes are found within macromolecules such as Complex III and Complex IV. They also function as electron carriers, but in a very different, intramolecular, solid-state environment.
Electrons may enter an electron transport chain at the level of a mobile cytochrome or quinone carrier. For example, electrons from inorganic electron donors (nitrite, ferrous iron, etc.) enter the electron transport chain at the cytochrome level. When electrons enter at a redox level greater than NADH, the electron transport chain must operate in reverse to produce this necessary, higher-energy molecule.
[edit] Terminal oxidases and reductases
When bacteria grow in aerobic environments, the terminal electron acceptor (O2) is reduced to water by an enzyme called an oxidase. When bacteria grow in anaerobic environments, the terminal electron acceptor is reduced by an enzyme called a reductase.In mitochondria the terminal membrane complex (Complex IV) is cytochrome oxidase. Aerobic bacteria use a number of different terminal oxidases. For example, E. coli does not have a cytochrome oxidase or a bc1 complex. Under aerobic conditions, it uses two different terminal quinol oxidases (both proton pumps) to reduce oxygen to water.
Anaerobic bacteria, which do not use oxygen as a terminal electron acceptor, have terminal reductases individualized to their terminal acceptor. For example, E. coli can use fumarate reductase, nitrate reductase, nitrite reductase, DMSO reductase, or trimethylamine-N-oxide reductase, depending on the availability of these acceptors in the environment.
Most terminal oxidases and reductases are inducible. They are synthesized by the organism as needed, in response to specific environmental conditions.
[edit] Electron acceptors
Just as there are a number of different electron donors (organic matter in organotrophs, inorganic matter in lithotrophs), there are a number of different electron acceptors, both organic and inorganic. If oxygen is available, it is invariably used as the terminal electron acceptor, because it generates the greatest Gibbs free energy change and produces the most energy.In anaerobic environments, different electron acceptors are used, including nitrate, nitrite, ferric iron, sulfate, carbon dioxide, and small organic molecules such as fumarate.
Since electron transport chains are redox processes, they can be described as the sum of two redox pairs. For example, the mitochondrial electron transport chain can be described as the sum of the NAD+/NADH redox pair and the O2/H2O redox pair. NADH is the electron donor and O2 is the electron acceptor.
Not every donor-acceptor combination is thermodynamically possible. The redox potential of the acceptor must be more positive than the redox potential of the donor. Furthermore, actual environmental conditions may be far different from standard conditions (1 molar concentrations, 1 atm partial pressures, pH = 7), which apply to standard redox potentials. For example, hydrogen-evolving bacteria grow at an ambient partial pressure of hydrogen gas of 10-4 atm. The associated redox reaction, which is thermodynamically favorable in nature, is thermodynamically impossible under “standard” conditions.
[edit] Summary
Bacterial electron transport pathways are, in general, inducible. Depending on their environment, bacteria can synthesize different transmembrane complexes and produce different electron transport chains in their cell membranes. Bacteria select their electron transport chains from a DNA library containing multiple possible dehydrogenases, terminal oxidases and terminal reductases. The situation is often summarized by saying that electron transport chains in bacteria are branched, modular, and inducible.[edit] Photosynthetic electron transport chains
In oxidative phosphorylation, electrons are transferred from a high-energy electron donor (e.g., NADH) to an electron acceptor (e.g., O2) through an electron transport chain. In photophosphorylation, the energy of sunlight is used to create a high-energy electron donor and an electron acceptor. Electrons are then transferred from the donor to the acceptor through another electron transport chain.Photosynthetic electron transport chains have many similarities to the oxidative chains discussed above. They use mobile, lipid-soluble carriers (quinones) and mobile, water-soluble carriers (cytochromes, etc.). They also contain a proton pump. It is remarkable that the proton pump in all photosynthetic chains resembles mitochondrial Complex III.
Photosynthetic electron transport chains are discussed in greater detail in the articles Photophosphorylation, Photosynthesis, Photosynthetic reaction center and Light-dependent reaction.
[edit] Summary
Electron transport chains are redox reactions that transfer electrons from an electron donor to an electron acceptor. The transfer of electrons is coupled to the translocation of protons across a membrane, producing a proton gradient. The proton gradient is used to produce useful work.The coupling of thermodynamically favorable to thermodynamically unfavorable biochemical reactions by biological macromolecules is an example of an emergent property – a property that could not have been predicted, even given full knowledge of the primitive geochemical systems from which these macromolecules evolved.[original research?] It is an open question whether such emergent properties evolve only by chance, or whether they necessarily evolve in any large biogeochemical system, given the underlying laws of physics.[citation needed]
[edit] Notes
- ^ Karp discusses ATP gradient: "These three protein complexes [I, II, and IV] are often described as proton pumps. The translocation of protons by these electron-transporting complexes establishes the proton gradient that drives ATP synthesis."(194)
- ^ Harper's Illustrated Biochemistry explaining the function of the complexes of the transport chain: "Each of the respiratory chain complexes I, II, and IV... acts as a proton pump...creating an electrochemical potential difference across the [mitochondrial inner] membrane."(96)
Krebs Cycle
Citric acid cycle
From Wikipedia, the free encyclopedia
(Redirected from Krebs cycle)
Jump to: navigation, search
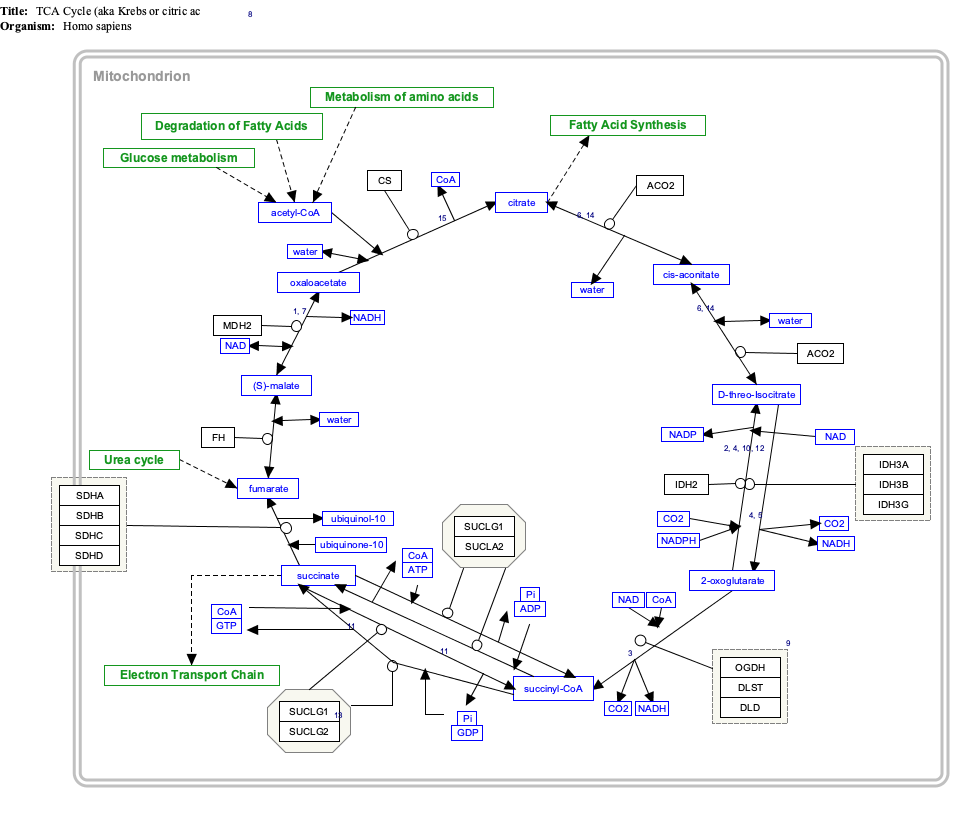
The citric acid cycle — also known as the tricarboxylic acid cycle (TCA cycle), the Krebs cycle, or the Szent-Györgyi-Krebs cycle, [1][2] — is a series of enzyme-catalysed chemical reactions, which is of central importance in all living cells that use oxygen as part of cellular respiration. In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. The components and reactions of the citric acid cycle were established by seminal work from Albert Szent-Györgyi and Hans Krebs.In aerobic organisms, the citric acid cycle is part of a metabolic pathway involved in the chemical conversion of carbohydrates, fats and proteins into carbon dioxide and water to generate a form of usable energy. Other relevant reactions in the pathway include those in glycolysis and pyruvate oxidation before the citric acid cycle, and oxidative phosphorylation after it. In addition, it provides precursors for many compounds including some amino acids and is therefore functional even in cells performing fermentation.
Contents[hide] |
[edit] A simplified view of the process
- The citric acid cycle begins with the transfer of a two-carbon acetyl group from acetyl-CoA to the four-carbon acceptor compound (oxaloacetate) to form a six-carbon compound (citrate).
- The citrate then goes through a series of chemical transformations, losing two carboxyl groups as CO2. The carbons lost as CO2 originate from what was oxaloacetate, not directly from acetyl-CoA. The carbons donated by acetyl-CoA become part of the oxaloacetate carbon backbone after the first turn of the citric acid cycle. Loss of the acetyl-CoA-donated carbons as CO2 requires several turns of the citric acid cycle. However, because of the role of the citric acid cycle in anabolism, they may not be lost, since many TCA cycle intermediates are also used as precursors for the biosynthesis of other molecules.[3]
- Most of the energy made available by the oxidative steps of the cycle is transferred as energy-rich electrons to NAD+, forming NADH. For each acetyl group that enters the citric acid cycle, three molecules of NADH are produced.
- Electrons are also transferred to the electron acceptor Q, forming QH2.
- At the end of each cycle, the four-carbon oxaloacetate has been regenerated, and the cycle continues.
[edit] Steps
Two carbon atoms are oxidized to CO2, the energy from these reactions being transferred to other metabolic processes by GTP (or ATP), and as electrons in NADH and QH2. The NADH generated in the TCA cycle may later donate its electrons in oxidative phosphorylation to drive ATP synthesis; FADH2 is covalently attached to succinate dehydrogenase, an enzyme functioning both in the TCA cycle and the mitochondrial electron transport chain in oxidative phosphorylation. FADH2, therefore, facilitates transfer of electrons to coenzyme Q, which is the final electron acceptor of the reaction catalyzed by the Succinate:ubiquinone oxidoreductase complex, also acting as an intermediate in the electron transport chain.[4]The citric acid cycle is continuously supplied with new carbon in the form of acetyl-CoA, entering at step 1 below.[5]
| Substrates | Products | Enzyme | Reaction type | Comment | |
|---|---|---|---|---|---|
| 1 | Oxaloacetate + Acetyl CoA + H2O | Citrate + CoA-SH | Citrate synthase | Aldol condensation | rate-limiting stage (irreversible), extends the 4C oxaloacetate to a 6C molecule |
| 2 | Citrate | cis-Aconitate + H2O | Aconitase | Dehydration | reversible isomerisation |
| 3 | cis-Aconitate + H2O | Isocitrate | Hydration | ||
| 4 | Isocitrate + NAD+ | Oxalosuccinate + NADH + H + | Isocitrate dehydrogenase | Oxidation | generates NADH (equivalent of 2.5 ATP) |
| 5 | Oxalosuccinate | α-Ketoglutarate + CO2 | Decarboxylation | irreversible stage, generates a 5C molecule | |
| 6 | α-Ketoglutarate + NAD+ + CoA-SH | Succinyl-CoA + NADH + H+ + CO2 | α-Ketoglutarate dehydrogenase | Oxidative decarboxylation | irreversible stage, generates NADH (equivalent of 2.5 ATP), regenerates the 4C chain (CoA excluded) |
| 7 | Succinyl-CoA + GDP + Pi | Succinate + CoA-SH + GTP | Succinyl-CoA synthetase | substrate-level phosphorylation | or ADP→ATP instead of GDP→GTP,[4] generates 1 ATP or equivalent |
| 8 | Succinate + ubiquinone (Q) | Fumarate + ubiquinol (QH2) | Succinate dehydrogenase | Oxidation | uses FAD as a prosthetic group (FAD→FADH2 in the first step of the reaction) in the enzyme,[4] generates the equivalent of 1.5 ATP |
| 9 | Fumarate + H2O | L-Malate | Fumarase | H2O addition (hydration) | |
| 10 | L-Malate + NAD+ | Oxaloacetate + NADH + H+ | Malate dehydrogenase | Oxidation | generates NADH (equivalent of 2.5 ATP) |
The GTP that is formed by GDP-forming succinyl-CoA synthetase may be utilized by nucleoside-diphosphate kinase to form ATP (the catalyzed reaction is GTP + ADP → GDP + ATP).[4]
[edit] Products
Products of the first turn of the cycle are: one GTP (or ATP), three NADH, one QH2, two CO2.Because two acetyl-CoA molecules are produced from each glucose molecule, two cycles are required per glucose molecule. Therefore, at the end of two cycles, the products are: two GTP, six NADH, two QH2, and four CO2
| Description | Reactants | Products |
| The sum of all reactions in the citric acid cycle is: | Acetyl-CoA + 3 NAD+ + Q + GDP + Pi + 2 H2O | → CoA-SH + 3 NADH + 3 H+ + QH2 + GTP + 2 CO2 |
| Combining the reactions occurring during the pyruvate oxidation with those occurring during the citric acid cycle, the following overall pyruvate oxidation reaction is obtained: | Pyruvate ion + 4 NAD+ + Q + GDP + Pi + 2 H2O | → 4 NADH + 3 H+ + QH2 + GTP + 3 CO2 |
| Combining the above reaction with the ones occurring in the course of glycolysis, the following overall glucose oxidation reaction (excluding reactions in the respiratory chain) is obtained: | Glucose + 10 NAD+ + 2 Q + 2 ADP + 2 GDP + 4 Pi + 2 H2O | → 10 NADH + 10 H+ + 2 QH2 + 2 ATP + 2 GTP + 6 CO2 |
The total number of ATP obtained after complete oxidation of one glucose in glycolysis, citric acid cycle, and oxidative phosphorylation is estimated to be between 30 and 38. A recent assessment of the total ATP yield with the updated proton-to-ATP ratios provides an estimate of 29.85 ATP per glucose molecule. [8]
[edit] Regulation
Although pyruvate dehydrogenase is not technically a part of the citric acid cycle, its regulation is included here.The regulation of the TCA cycle is largely determined by substrate availability and product inhibition. NADH, a product of all dehydrogenases in the TCA cycle with the exception of succinate dehydrogenase, inhibits pyruvate dehydrogenase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and also citrate synthase. Acetyl-coA inhibits pyruvate dehydrogenase, while succinyl-CoA inhibits succinyl-CoA synthetase and citrate synthase. When tested in vitro with TCA enzymes, ATP inhibits citrate synthase and α-ketoglutarate dehydrogenase; however, ATP levels do not change more than 10% in vivo between rest and vigorous exercise. There is no known allosteric mechanism that can account for large changes in reaction rate from an allosteric effector whose concentration changes less than 10% [9].
Calcium is used as a regulator. It activates pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase.[10] This increases the reaction rate of many of the steps in the cycle, and therefore increases flux throughout the pathway.
Citrate is used for feedback inhibition, as it inhibits phosphofructokinase, an enzyme involved in glycolysis that catalyses formation of fructose 1,6-bisphosphate,a precursor of pyruvate. This prevents a constant high rate of flux when there is an accumulation of citrate and a decrease in substrate for the enzyme.
Recent work has demonstrated an important link between intermediates of the citric acid cycle and the regulation of hypoxia-inducible factors (HIF). HIF plays a role in the regulation of oxygen homeostasis, and is a transcription factor that targets angiogenesis, vascular remodeling, glucose utilization, iron transport and apoptosis. HIF is synthesized consititutively, and hydroxylation of at least one of two critical proline residues mediates their interaction with the von Hippel Lindau E3 ubiquitin ligase complex, which targets them for rapid degradation. This reaction is calalysed by prolyl 4-hydroxylases. Fumarate and succinate have been identified as potent inhibitors of prolyl hydroxylases, thus leading to the stabilisation of HIF.[11]
[edit] Major metabolic pathways converging on the TCA cycle
Several catabolic pathways converge on the TCA cycle. Reactions that form intermediates of the TCA cycle in order to replenish them (especially during the scarcity of the intermediates) are called anaplerotic reactions.The citric acid cycle is the third step in carbohydrate catabolism (the breakdown of sugars). Glycolysis breaks glucose (a six-carbon-molecule) down into pyruvate (a three-carbon molecule). In eukaryotes, pyruvate moves into the mitochondria. It is converted into acetyl-CoA by decarboxylation and enters the citric acid cycle.
In protein catabolism, proteins are broken down by proteases into their constituent amino acids. The carbon backbone of these amino acids can become a source of energy by being converted to acetyl-CoA and entering into the citric acid cycle.
In fat catabolism, triglycerides are hydrolyzed to break them into fatty acids and glycerol. In the liver the glycerol can be converted into glucose via dihydroxyacetone phosphate and glyceraldehyde-3-phosphate by way of gluconeogenesis. In many tissues, especially heart tissue, fatty acids are broken down through a process known as beta oxidation, which results in acetyl-CoA, which can be used in the citric acid cycle. Beta oxidation of fatty acids with an odd number of methylene groups produces propionyl CoA, which is then converted into succinyl-CoA and fed into the citric acid cycle.[12]
The total energy gained from the complete breakdown of one molecule of glucose by glycolysis, the citric acid cycle, and oxidative phosphorylation equals about 30 ATP molecules, in eukaryotes. The citric acid cycle is called an amphibolic pathway because it participates in both catabolism and anabolism.
[edit] Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [13] Citric acid cycle [edit]
Subscribe to:
Posts (Atom)